
(Wikilink:Exosomes) - (01/01/2024)
This page is currently still being developed and some elements may not be completed.
The Emergence of Exosomes into the
Age-Regression Race
Click [√] to Enlarge
Exosomes are a type of extracellular vesicles (EVs), very tiny particles released by cellular membranes into the extracellular space. Exosomes range between 30 and 150 nanometers (nm). To put this into perspective, the average diameter of a human hair ranges from about 50,000 to 100,000 nanometers. This means that an exosome is about 500 to 3,000 times smaller than the width of a human hair. Exosomes are so small only the most advanced microscopes can visualize them.
Click [√] to Enlarge. Source: [2021] Exosomal therapy—a new frontier in regenerative medicine.pdf
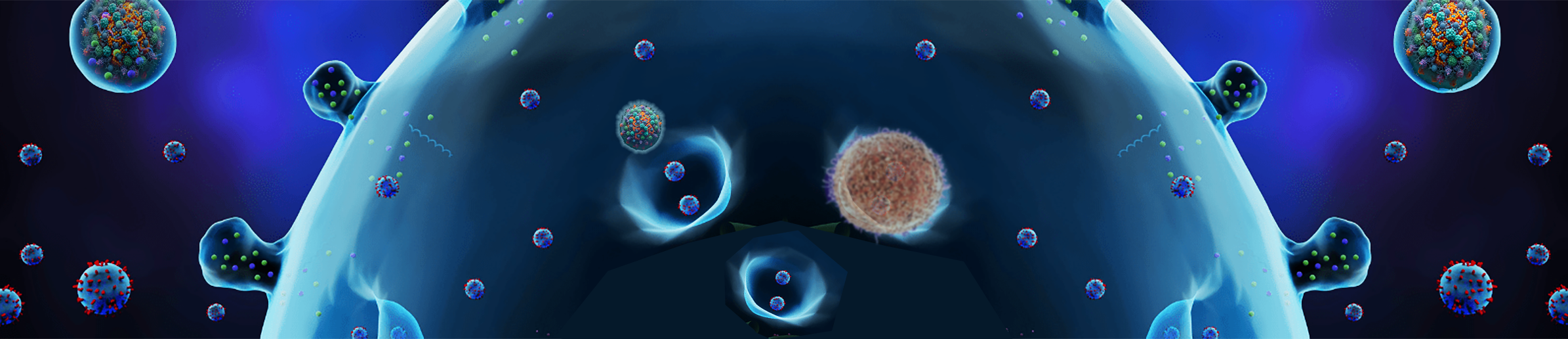
Exosomes provide a critical cell-to-cell communication capability. Most of the cells contained within the body are sequestered in a tissue matrix, locking them in place. As the illustration (below left) details, exosomes both exit and enter cells enabling a send and receive signaling process emanating from both membrane bound receptors and bi-lipid contained cargos. This is facilitated by four types of proteins embedded in the bi-lipod membrane: tetraspanins, adhesion proteins, antigen presentation proteins and membrane transport and fusion protein. Because the majority of the cells don’t move, cellular expressed exosomes provide an ability to transit messages they deem important to any other cells in the body. Additionally exosome are capable of crossing all the body barriers, including the blood brain barrier.
Click [√] to Enlarge
Dramatic restorative, disease ameliorative, and age regressive benefits have been demonstrated by administering exosomes in both animal and human studies. The therapeutic potential of what was once thought to be the trash removal system of cells, is now considered to be virtually unlimited. This is, in part due to the complexity of exosomes, as they carry a multitude of signaling moieties, including proteins, lipids, cell surface receptors, enzymes, cytokines, transcription factors, and nucleic acids. Nuclecic acids in the form of messenger RNA (mRNA) may provide the most important cargo as they enable the exosome to direct rapid protein production in neighboring cells without the need to initiate or activate genes harbored within the DNA. 2021, Brian V. Lananna and Shin-ichiro Imai described the emerging evidences as suggesting that EVs regulate systemic aging as well as the pathophysiology of age-related diseases. The illustration on the right is from their paper. [14]
Currentely there are about 200 studies listed on ClinicalTrails.Gov incorporating exosomes and over 100 companies developing these strategies.
An Exosome Analogy: The Biological Internet
Imagine the human body as a vast, intricate network, akin to the map on the left depicting the global internet (Click to Enlarge). This network is bustling with communication, not in the form of digital data, but through biological signals. The cells, like computers, are the fundamental units in this network, each endowed with its own unique 'address' – surface receptors that identify them and facilitate communication.
Exosomes: Biological Data Packets
In the realm of biological systems, exosomes function similarly to data packets in the Internet Protocol (IP). These tiny vesicles or biological packets, released by cells into the extracellular space, carry molecular information such as proteins, lipids, and RNA, akin to how data packets transport information from one computer to another, across the internet. Once an exosome reaches its target cell, akin to a data packet reaching its destined IP address, it delivers its cargo.
This addressing system allows exosomes to be recognized and absorbed by cells with corresponding receptors throughout the biological system they reside within. Upon reaching the target cell, exosomes deliver their cargo, influencing the recipient cell's behavior and those in close proximity to it, thereby initiating various biological processes. This parallels how data packets trigger specific actions in computers. Their journey through the body, influenced by factors like concentration gradients and tissue barriers, mirrors the routing of data packets in the internet, guided by protocols, routers and network conditions. This analogy not only illuminates the complex communication within biological systems but also highlights the potential for manipulating exosomes in biomedical research. By selecting or modifying their 'biological IP addresses' and cargos, researchers can target specific cells, paving the way for novel, targeted therapies in conditions like diseases associated with aging, infections and aging itself.
The current estimate of the number of nodes or computers currently running and connected to the internet is between 40 to 50 billion. Studies indicate that the plasma of a healthy individual typically contains approximately 1 billion (1x10^9) to 1 trillion (1x10^12) exosomes per milliliter. Considering an average adult has about 5 liters (5,000 milliliters) of blood, this equates to an estimated total of 5 trillion to 5 quadrillion exosomes circulating in the body at any given moment.
An excellent paper was recientely published describing: Exosomes, as the Key Players in Cell-To-Cell Communication and Universal Nano-Sized Disease Sensors of the Future. [2023] Exosomes, the Key Players in Cell-To-Cell Communication as the Universal Nano-Sized Disease Sensors of the Future
Exosomes Defined
Because every cell is capable of expressing exosomes, the therapeutic profile of each is highly variable. Small environmental changes or changes in the health and disease state of the expressing cell can also dramatically change the signaling cargos of exosomes. Additionally and importantly from the perspective of this web sites focus, the expression of those signals change as the organism ages. Exosomes derived from young cells communicate youthful signals and are capable of regressing the age of older animals. Older organisms express exosomes that can actually age a young animal when infused. This bidirectional communication highlights the significant role of exosomes in conveying age-related information and their potential in therapeutic applications for age regression.
Exosomes have the ability to redefine the health and age state of the cells they enter. This makes them powerful disease fighting and age amerolating biologics. Some of these treatments will become accessible in a few months by volunteering for clinical trails that will test their safety and therapeutic profile. Some groups have indicated that they could be commercially available in 12 to 18 months. For biohackers and individuals not adverse to an increased risk profile, some exosomes with a still emerging therapeutic profiles are available now. The therapeutic, safety and risk profiles are still emerging although no allergic reaction have been. Ascetics targeting clinics have been routinely incorporating topically applied exosomes for several years.
Since few transplanted cells persist in vivo, the beneficial effects of cell therapy may lie in the secreted factors being the active component of this treatment. A key part of paracrine secretions are exosomes, which are membrane vesicles that are stored intracellularly in endosomal compartments and are secreted when these structures fuse with the cell plasma membrane.
Therapeutic Advantages of Exosomes
Comparable therapeutic effects to stem cells
Concentrated functional cargos: cytokines, mRNAs
Modifiable surface receptors and cargos
Stable long-term storage and transportation
Greatly reduced risk of tumorigenesis and immune response
Lack of ethical issues
While the potential of exosomes as a therapy for age regression may seem far-fetched to those unfamiliar with the historical context, a closer look at the scientific journey leading up to this point reveals a series of groundbreaking advancements. The brief history provided below traces each critical step in the evolution of aging research, showing how each discovery has built upon the last, culminating in recent, seemingly unbelievable breakthroughs. For those new to this field, understanding this progression is key to appreciating and believing the current state of research, where the prospect of targeting age as a treatable condition is not only plausible but rapidly approaching.
From Myth to Modern Science:
The Rejuvenating Power of Young Blood
Throughout history, the quest for immortality has been a persistent theme in human culture, often intertwined with the mystical allure of young blood. Ancient civilizations (circa 3000 BCE - 476 CE) revered blood, especially from the young, for its supposed life-giving properties. This concept found its way into various cultures and epochs, most notably through the enduring legends of vampires. From ancient times to the present, these tales depicted immortality achieved through blood consumption, reflecting humanity's deep-seated fascination with and fear of mortality.
During the Medieval and Renaissance periods (circa 476 - 17th century), this fascination took a more practical turn. Alchemists and early physicians speculated on the restorative properties of blood, a pursuit that mirrored the broader search for the elixir of life. Although steeped in mysticism and often lacking empirical evidence, these early explorations hinted at a profound truth that modern science is only now beginning to uncover.
In an ironic twist of fate, contemporary scientific research has started to validate what these ancient myths and practices have long suggested. Studies in parabiosis, particularly involving the circulatory systems of young and old mice, have revealed that factors present in young blood can indeed rejuvenate older organisms. This groundbreaking research suggests that certain elements in young blood , primarily exosomes at this juncture, have the potential to reverse or slow down some aspects of the aging process.
This convergence of myth and science is a remarkable testament to the intuitive understanding of our ancestors. It underscores a fascinating aspect of human knowledge: myths, often dismissed as mere fabrications, can unexpectedly contain kernels of valid scientific truth. In the case of the rejuvenating power of young blood, a concept once shrouded in legend and mystique, we find a striking example of how ancient beliefs and modern scientific discoveries can align, revealing insights that have been discounted for centuries.
Parabiosis: The first Clues to Aging Plasticity
Click [√] to Enlarge
Humanity's enduring quest to conquer diseases, slow or reverse aging, and evade death has led to significant scientific breakthroughs. A notable milestone was achieved over a century ago with the introduction of heterochronic parabiosis, a novel technique that combines the vascular systems of young and old animals. (see illustration on right).
The first recorded experiment of parabiosis dates back to 1864 by the French physiologist Paul Bert. Bert's doctoral thesis, in which he described the concept of combining the vascular systems of two animals, is titled "Sur la Greffe Animale." In this thesis, Bert detailed his experiments on albino rats, where he sutured the skin of two rats at their flanks and observed that intravenously administered fluids passed from the circulation of one animal into the bloodstream of its attached partner. [1]
Source: [2014] A Revival of Parabiosis in Biomedical Research Click [√] to Enlarge
A pioneering parabiosis study by Clive McCay at Cornell University in 1957 [2], joined the circulatory systems of young and old rats, providing early evidence of the rejuvenating potential of young blood, observed through improvements in cartilage health and other age-related conditions in older rats. In 1972, Ludwig and Elashoff, at the University of California studied the lifespans of old, young rat pairs. Older partners conjoined to young rats lived for four to five months longer than controls, suggesting for the first time that the circulation of young blood might affect longevity.[3] This provided the first documented study resulting in the extension of life in an old animal as a result of exposure to a young animals blood.
In 2005, researchers Irena Conboy and Thomas Rando reinvigorated interest in parabiosis, exploring in greater depth the molecular and cellular mechanisms through which young blood might rejuvenate older organisms and its potential implications for human aging and age-related diseases.[4] This ignited a quest to identify exactly what the signaling molecules were within the blood that imparted the age regression benefits. Harold Ketcher was the first to have practical scientific insights into exactly what these age directing factors were. [10][16][19]
Tony Wyss-Coray's group previously identified 529 proteins that have been reported by multiple studies derived from 4263 healthy individuals with an age range of 18-95 years. A total of 529 proteins changed their expression level with age in human plasma. A literature search revealed that at least 64 of these 529 proteins are capable of regulating life span in animal models. They also determined that: (75.84%) 361 of all proteins associated with aging increased expression with age, while only (24.16%) 115 are associated with a decreased expression. Wyss-Coray has referred to these age signaling molecules as Chronokines. Chrono indicating time and kines indicating signaling. It would appears obvious that modulating the increasing signals to a lower level and increasing the declining signals to a higher level would be an effective interventional stratgie. One study by the Conboys demonstrated the benefits of this type of intervention. [6]
Heterochronic parabiosis has been instrumental in uncovering many of the secrets of aging. It disclosed the potential of young mammals' plasma, which contains age signaling molecules. Youthful signals are referred to here a Type 1 Chronokine Profiles (T1CP). Aged or old signals are identified as Type 2 Chronokine Profiles (T2CP). The levels of each age-defining molecule present in the plasma of younger organisms, capable of imparting anti-aging effects when introduced in sufficient quantities into older animals, essentially represent a youthful biochemical signature that defines and aligns the cells of the older organism to the same approximate age as the donor.
◎ Parabiotic studies are continuing to demonstrate multiple benefits of pairing young and old animals, particularly overloading the system with youthful, T1CP signals, overridingand/or suppressing T2CP aging signals. [13] These benefits include enhanced muscle and tissue regeneration, mirroring younger counterparts, marked by epigenetic clock alterations. Cognitive functions, including memory and learning, improve, while signs of cardiac aging, such as heart hypertrophy, are reduced. Liver function is notably better, and stem cell revitalization occurs, aiding in tissue maintenance and repair. There's a significant reduction in age-related biomarkers, suggesting a systemic reversal of aging, including dramatic reductions in multiple epigenetic clocks. Additionally, age-related diseases, including degenerative conditions, show signs of alleviation or resolution. Finally, an increase in lifespan has been observed in older animals, attributed to the rejuvenating effects of young blood.
As we indicated, these studies are continuing today with the most recent one [Biological Age Reduction and Lifespan Extension Upon Youthful Circulation Exposure; Zang et al., 2023] demonstrating that the two most effective age-regression targets: epigenetic and transcriptome remodeling, are both modulated in a positive way resulting in the extension of lifespan and health'span.
Another important insight was again accomplished by the Conboy’s when they demonstrated that simply diluting the plasma of older (T2CP) animals with sterile saline provided significant benefits in multiple tissue types, improved memory and cognition and reduced inflammation. This suggests that a mere reduction in the T2CP signals is sufficient to improve the health of an individual. An approved medical procedure, therapeutic plasma exchange or plasmapheresis is currently available to achieve this type of plasma dilution. A large human clinical trial is currently being conducted by Dobri D. Kiprov, MD HP (ASCP) In collaboration with Irena Conboy’s group to determine the impact on aging and disease from the dilution of the plasma by plasmapheresis.[9]
In 2020 it was determined by Harold Katcher [10] that fractions within the plasma as opposed to the blood cells contained the molecular signaling agents responsible for these biological, age signaling benefits. Although identified in his first patent[16], it was not until a 2023 publication that the active molecules imparting age regressive benefits were definitively identified as a specific subtype of extracellular vesicles; exosomes.[19].

A 2023 Comprehensive Examination of Exosomes by: International Society for Extracellular Vesicles (ISEV)
ISEV updates its ‘Minimal Information for Studies of Extracellular Vesicles’ (MISEV), which was first published in 2014. The goal of the current document, MISEV24, is to provide researchers with an updated snapshot of available approaches and their advantages and limitations for Production, Separation and Characterisation of EVs from multiple sources, including cell culture, body fluids and solid tissues. In addition to presenting the latest state of the art in basic principles of EV research, this document also covers advanced techniques and approaches that are currently expanding the boundaries of the field.

Notable Studies Supporting Exosomes as Age-Regressive & Regenerative Therapeutics
Most published studies incorporating exosomes have been in animal models. Some examples of the efficacy of exosomes in humans are N of 1, but still provide dramatic evidence of the potential benefits of exosomes.
Overview of the Benefits Seen in the Study by Harold Katcher: "Reversal of biological age in multiple rat organs by [Exosomes] young porcine plasma fraction"
The most notable study incorporating exosomes was conducted by Harold Katcher. This cross-species study demonstrated a significant epigenetic reduction in age of the rats receiving the infusions.
• 67.4% reduction in epigenetic age in blood
• 74.6% in liver
• 46.5% in heart tissue
• 24.4% in the hypothalamus
The study also dramatically demonstrates the non-immunogenic nature of exosomes. Exosomes represent a stealth encapsulation of the aging and disease ameralating cargos they deliver directly into targeted cells. By harvesting exosomes expressed by the entire resident cellular population, the resulting therapeutic captures the entire signaling/information signature of the young animal and effectively transfers that signaling milieu to the older animal. The study utilized the exosomes isolated from the plasma of young pigs, 6-7 months old, just prior to reaching puberty.
Enhanced Learning Ability: The treatment led to an increase in learning ability in older rats, as evidenced by a decrease in the time spent on a Barnes maze. This improvement was comparable to that observed in younger control animals.
Increased Muscle Strength: A significant increase in muscle strength was observed in the treated older group, as measured by a grip strength meter. The muscle strength in the treated old group was comparable to that of the young control group.
Improved Liver and Kidney Function: Biochemical tests showed better liver and kidney function in the treated old group compared to the old vehicle control group, after 8 days of treatment.
Increased Antioxidant Levels: The treatment resulted in increased levels of reduced glutathione (GSH) in various organs such as the brain, heart, lung, and liver. This indicates enhanced antioxidant capacity.
Elevated Catalase Activity: The catalase activity, which is another indicator of antioxidant defense, was found to be higher in the treated old group across different organs, including the brain, heart, lung, and liver.
Enhanced Superoxide Dismutase (SOD) Activity: There was an increase in SOD activity in the treated old group. SOD is crucial for dismuting superoxide radicals, a type of reactive oxygen species.
Reduced Lipid Peroxidation: The treatment reduced lipid peroxidation, as indicated by lower malondialdehyde (MDA) levels, in organs like the brain, heart, lung, and liver. This suggests a reduction in oxidative stress damage.
Increased Nrf2 Levels: Rat nuclear factor erythroid 2-related factor 2 (Nrf2), a key regulator of antioxidant response, was found to be increased in the treated old group across multiple organs.
Decreased Inflammatory Markers: There was a remarkable decrease in the concentration of Interleukin-6 (IL-6) in the treated old group, bringing the levels closer to those observed in the young control group.
Reduction in TNF Alpha: The concentration of Tumor Necrosis Factor (TNF) Alpha significantly decreased in the treated old group compared to the old control group after 30 days of treatment.
These results suggest that exosomal plasma fraction treatments can effectively reverse certain age-related physiological changes in rats, improving various biomarkers associated with aging and health. If you compare these results with those identified in parabosisi studies you will note a strikingly similar therapeutic profile. Look for this bullet icon above ◎.
One notable antidotal outcome emanating from this study was the principal investigator applied some left over exosomes utilized in the study to his right hand. Dr. Katcher is in his eighties and as you can see the results are dramatic. In comparison to the other images we provide below it should be noted that the exosome utilized in his study were derived from all of the cells in circulation of the young pigs. In contrast, all other images demonstrate the benefits of exosomes obtained from a single cell line.
Click [√] to Enlarge
Kimera Labs has been involved in the isolation, production and research of exosomes beginning in 2012. The cell source they focus on are immortalized placental, Mesenchymal Stem Cells (pMSC). The companies information is provided in the Directory of Companies. provided on the next page.
Duncan Ross’s, Ph.D., and his group have made several important advances in both exosome production methods and important contributions to understanding the potential of exosomes as therapeutics.
Below is a dramatic example of the potential of placental derived exosomes. This individual was spared from the disfiguring scars normally associated with severe burns. Because of the embryonic type rejuvenation the exosomes provided to this individual, the health and complexion of his face was actually better, after the burns and the subsequent treatment than before the accident.
Click [√] to Enlarge Image Source: [2020] Hydrogel Dressings for the Treatment of Burn Wounds: An Up-To-Date Overview
This series of photos demonstrates the rescue potential of Kimera’s placental derived exosomes in preventing the formation of scar tissue in a second degree burn patent. The severity of second degree burns should not be underestimated as indicated but the graphic on the right. The ExoGlo exosomes that were administered to this individual were derived from conditioned medium, that also contained the growth factors and proteins contained within the cell culture they were obtained from. This exosome formulation was administers by spraying it on his face, three times a day for seven days.
Videos
To review videos describing Dr. Katcher’s work with E5 please go to this page: Katcher’s Procine Exosomes
One of the most educational and enlightening videos by Dr. Ross can be found: here
Cell Source Selection by Biological Response
Exosomes are secreted by almost all cell types including stem cells have been proposed as a safer and more versatile alternative to stem cell therapy. [Top of Illustration]
Exosomes have been regarded as miniature versions of their parental cells, partially because exosomes from a certain cell type provide unique, cell-specific sets of biomolecules. [Bottom of Illustration}
Exosomes, produced by virtually every type of cell, serve as a critical component of direct and unfiltered intercellular communication. Their wide biological responses are shaped both by the specific cell type from which they are derived and by various environmental factors impacting the cell.
Functioning in both paracrine and endocrine signaling, exosomes can affect nearby cells or those distant from their origin. They are packed with a diverse range of contents, including proteins (encompassing signaling, growth, and inflammatory factors), mRNA (which are blueprints for protein production), and microRNA (acting as an on-off switch for specific genes and protein production).
These molecular cargos, encapsulated within a membrane harvested from their parent cell during the budding process, confers upon exosomes the unique biological capabilities of the cells they were produced by.
Collectively, stem cell-derived exosomes are a potent surrogate for stem cell therapy without exhibiting the disadvantages their cellular counterparts.
Cell Types (Top) Isolation (Middle) and Potential Exosome Cargos (Bottom)
Click [√] to Enlarge. Source: [2024] Clinical applications of stem cell-derived exosomes [22]
As a result, they play pivotal roles in influencing various biological processes, including aging, tissue regeneration, immune modulation, and cellular differentiation, tailored according to the nature of their origin and the specific molecular signals they carry.

Exosome Biological Attributes by Cell Source
The following synopsis's provides a general envelope of functionality based on the cell source of the exosomes.
Their influence on cancer progression is multifaceted, as they can modulate the tumor microenvironment, mediate immune responses, and contribute to the processes of angiogenesis, metastasis, and drug resistance. The impact of exosomes on cancer risk and progression can vary significantly depending on their cellular origin. Additionally, growth factors and promoters that can facilitate the growth of cancers are part of the cargo contents of many exosomes. Cancer in relation to each exosome type is an emerging arena and there is little actual bench top or clinical evidence of the risk. Potential cancer implications by each exsome type are listed in red text.
These overviews should not be utilized as a treatment guide, but as a resource to narrow the focus of your own due-diligence research.
◎ Adipose-Derived Exosomes
Exosomes from adipose tissue are known for their roles in tissue regeneration and wound healing. They carry signaling molecules that promote angiogenesis, the growth of new blood vessels, which is crucial for repairing damaged tissues. Additionally, these exosomes have anti-inflammatory properties and can modulate the immune response, making them potential candidates for treating chronic inflammatory diseases. Their content is also reflective of metabolic processes, linking them to metabolic regulation and possibly obesity-related disorders.
- Enhanced wound healing and tissue repair.
- Modulation of inflammatory responses.
- Potential role in metabolic regulation.
- Promote Tumor Growth: Can enhance tumor progression and metastasis by transferring oncogenic proteins and miRNAs to cancer cells.
- Immune Modulation: May modulate immune responses in the tumor microenvironment, potentially supporting immune evasion by tumor cells.
◎ Amniotic-Derived Exosomes
Exosomes from amniotic fluid play a vital role in fetal development and maternal-fetal tolerance. They are rich in growth factors and cytokines, contributing to tissue regeneration and repair. Their anti-inflammatory and immunomodulatory properties are crucial in maintaining pregnancy and preventing rejection of the fetus. These exosomes also carry information essential for the development of the fetal immune system and are being studied for their potential in regenerative medicine, particularly in wound healing and tissue repair applications.
- Promoting tissue regeneration and repair.
- Anti-inflammatory and immunomodulatory properties.
- Possible applications in fetal development and maternal-fetal tolerance.
- Anti-inflammatory: Often characterized by their anti-inflammatory properties, which might play a role in tumor suppression or promotion depending on the context.
- Tissue Repair and Regeneration: While primarily known for promoting tissue repair, their role in cancer is less clear and may involve modulation of the tumor microenvironment.
◎ Bone Marrow-Derived Exosomes
Exosomes from bone marrow are pivotal in supporting the growth and differentiation of hematopoietic cells. They play a significant role in bone and cartilage repair, which is of particular interest in orthopedics and regenerative medicine. These exosomes also possess immune-modulating properties, impacting both innate and adaptive immune responses. This characteristic makes them potential tools in treating autoimmune diseases and in modulating the body’s immune response to various pathological conditions. Additionally, their involvement in the regeneration of blood cells makes them a key focus in hematological research and therapies.
- Support of hematopoietic cell growth and differentiation.
- Involvement in bone and cartilage repair.
- Immune system modulation.
- Support for Tumor Growth: Can facilitate tumor growth and metastasis by promoting angiogenesis and suppressing immune surveillance.
- Communication Between Tumor and Bone Marrow: Play a key role in preparing the pre-metastatic niche and in the process of metastasis, especially in bone-related cancers.
◎ Embryonic Stem Cell-Derived Exosomes
Exosomes from embryonic stem cells are remarkable for their role in modulating developmental processes. Owing to the pluripotent nature of their cells of origin, these exosomes carry a wide array of signaling molecules that can influence cell differentiation and tissue development. They are involved in the regulation of various developmental pathways, making them integral to understanding embryogenesis. Their potential in regenerative medicine is significant due to their ability to promote cellular proliferation and differentiation. These exosomes could be key in developing therapies for degenerative diseases and in tissue engineering, given their role in directing stem cell fate and tissue regeneration.
- High potential in regenerative medicine due to pluripotency.
- Role in modulating embryonic development processes.
- Potential in modulating cellular differentiation.
- Promotion of Tumorigenesis: Potential to promote tumorigenesis due to their proliferative properties and the transfer of oncogenic factors.
- Regenerative Properties: While beneficial for tissue regeneration, these properties might inadvertently support tumor growth and progression.
◎ Induced Pluripotent Stem Cell (iPSC)-Derived Exosomes
Exosomes derived from induced Pluripotent Stem Cells (iPSCs) mirror many of the beneficial properties of embryonic stem cell-derived exosomes, with the added advantage of being patient-specific, reducing the risk of immune rejection. These exosomes are instrumental in cellular reprogramming and differentiation, carrying key regulatory molecules that guide the development of iPSCs into various cell types. This makes them particularly valuable in personalized regenerative medicine. iPSC-derived exosomes also show promise in neuroregeneration, potentially aiding in the repair of neural tissue damage, and are being explored for their role in treating various neurodegenerative disorders. Their capacity to deliver specific molecular signals to target cells without the risk of tumor formation (a concern with stem cell therapy) further enhances their therapeutic potential.
- Similar properties to embryonic exosomes, with regenerative and differentiation capabilities.
- Application in personalized medicine due to patient-specific cell source.
- Potential in neuroregeneration and repairing damaged tissues.
- Regenerative and Repair Mechanisms: Similar to embryonic stem cell-derived exosomes, with potential tumorigenic effects due to their ability to promote cell proliferation and survival.
- Potential for Reprogramming Tumor Cells: Theoretically could influence tumor behavior through the transfer of reprogramming factors, though the implications for cancer risk are complex and not fully understood.
◎ Multipotent Mesenchymal Stromal Cells (MSCs)-Derived Exosomes
Exosomes from Multipotent Mesenchymal Stromal Cells (MSCs) are highly regarded for their regenerative and immunomodulatory capabilities. These exosomes are involved in the repair and regeneration of various tissues, including bone, cartilage, and muscle, making them highly relevant in orthopedics and tissue engineering. MSC-derived exosomes have been shown to enhance wound healing and to possess anti-inflammatory properties, contributing to their potential in treating inflammatory and autoimmune diseases. Their role in modulating the immune system is particularly significant, as they can alter the behavior of immune cells, potentially leading to new treatments for immune-mediated conditions. Additionally, these exosomes' ability to promote angiogenesis and their involvement in tissue repair mechanisms make them promising candidates for cardiovascular and neurodegenerative disease therapies.
- Prominent role in tissue repair and regeneration.
- Immunomodulatory effects beneficial for treating autoimmune diseases.
- Potential in enhancing bone and cartilage repair.
- Dual Role in Cancer: Can either suppress or promote tumor growth depending on the tumor type and the context of their interaction with tumor cells.
- Immune Regulation: Capable of modulating the immune response, potentially influencing tumor immune evasion or immune-mediated tumor suppression.
◎ Placental-Derived Exosomes
Exosomes from placental tissue are integral to maternal-fetal communication and play a crucial role in pregnancy and fetal development. They carry a diverse array of signaling molecules, including growth factors and hormones, essential for fetal growth and development. Placental exosomes are also involved in modulating the maternal immune system to maintain pregnancy, preventing maternal immune rejection of the fetus. Their immunomodulatory properties are of particular interest in understanding pregnancy-related complications and immune tolerance. Additionally, these exosomes have been studied for their role in promoting angiogenesis and tissue repair, making them potential candidates for therapeutic use in regenerative medicine and wound healing.
- Significant role in fetal development and maternal-fetal exchange.
- Immunomodulatory properties important for maintaining pregnancy.
- Potential in promoting tissue repair and angiogenesis.
- Immunomodulatory Effects: Known for their role in maternal-fetal tolerance, these exosomes might influence cancer risk by modulating immune responses in the tumor microenvironment.
- Potential in Tumor Suppression: While research is limited, their unique immunomodulatory properties could be leveraged for therapeutic benefits in cancer.
◎ Platelet-Derived Exosomes
Exosomes derived from platelets are crucial in the processes of hemostasis, wound healing, and tissue regeneration. They carry a rich array of growth factors, cytokines, and other bioactive molecules that promote clot formation and wound healing. Platelet exosomes are involved in modulating the inflammatory response at injury sites, aiding in tissue repair and regeneration. Their role in angiogenesis is also significant, as they can promote the formation of new blood vessels, essential for healing damaged tissues. Given these properties, platelet-derived exosomes are being explored for their potential in treating cardiovascular diseases, enhancing wound healing, and in regenerative therapies, particularly for tissues with high vascularization needs.
- Key role in wound healing and tissue regeneration.
- Involvement in clot formation and inflammation modulation.
- Potential use in cardiovascular disease treatment and tissue repair.
- Promotion of Metastasis: Facilitate the spread of cancer by enhancing tumor cell migration, invasion, and angiogenesis.
- Cancer Cell Survival: Can protect circulating tumor cells from immune-mediated destruction, aiding in metastasis.
◎ Umbilical Cord-Derived Exosomes
Exosomes from umbilical cord tissue play a pivotal role in supporting fetal development, growth, and maternal-fetal communication. They are enriched with a wide array of growth factors, cytokines, and other signaling molecules that contribute to the development of fetal tissues. These exosomes also exhibit immunomodulatory properties, which are essential in maintaining the tolerance of the maternal immune system towards the fetus. Due to their rich content in growth factors and their regenerative properties, umbilical cord-derived exosomes are being explored in regenerative medicine. They show promise in enhancing tissue repair, promoting angiogenesis, and potentially treating various degenerative diseases. Their use is particularly attractive due to the non-invasive nature of their source and their relatively lower risk of eliciting an immune response.
- Role in supporting fetal development and growth.
- Potential in regenerative medicine due to rich growth factor content.
- Immunomodulatory properties relevant for preventing rejection in transplants.
- Regenerative Capabilities: While primarily studied for their regenerative and anti-inflammatory properties, the impact on cancer is an area of growing interest.
- Potential Anti-cancer Properties: Preliminary studies suggest they might carry anti-tumor cargoes, but further research is needed to fully understand their role in cancer.
◎ Conclusion
Each type of exosome, due to its unique origin, carries a distinct set of signaling molecules that can interact with recipient cells in different ways. This specificity makes them highly valuable in targeted therapies, where the goal is to modulate cellular processes in a precise manner. The study of these vesicles, especially in the context of regenerative medicine and aging, can provide insights into novel therapeutic approaches for a range of diseases, including age-related conditions.

References
[1][1863] Bert P. De la greffe animale
[2][1957] Parabiosis Between Old and Young Rats
[3][1972] Mortality in Syngeneic Rat Parabionts of Different Chronological Age
[4][2005] Rejuvenation of aged progenitor cells by exposure to a young systemic environment
[5][2010] Platelets Contribute to Allograft Rejection Through Glutamate Receptor Signaling
[7][2019] Exosomes: biogenesis, biologic function and clinical potential
[8][2019] Undulating changes in human plasma proteome profiles across the lifespan
[9][2020] Plasma dilution improves cognition and attenuates neuroinflammation in old mice
[10][2020] Reversing age- dual species measurement of epigenetic age with a single clock
[11][2020] Rejuvenation of three germ layers tissues by exchanging old blood plasma with saline-albumin
[12][2020] A Prospective Multicenter Longitudinal Observational Study to Evaluate the Efficacy and Safety of Long Term Therapeutic Plasma Exchange (TPE) for the Treatment and Prevention of Alzheimer’s Disease and other Age Related Medical Conditions.
[13][2021] Diverse plasma membrane protrusions act as platforms for extracellular vesicle shedding
[14][2021] Friends and foes: Extracellular vesicles in aging and rejuvenation (Introduction)
[15][2022] Old plasma dilution reduces human biological age - a clinical study
[16][2022] Anti-Aging Composition Use Thereof US 2022/0233587 A1
[17][2023] Multi-omic rejuvenation and lifespan extension on exposure to youthful circulation
[18][2023] Mechanism of mesenchymal stem cells and exosomes in the treatment of age-related diseases
[19][2023] Reversal of biological age in multiple rat organs by young porcine plasma fraction
[21][2023] Endothelial cells release microvesicles that harbor multivesicular bodies
[22][2024] Clinical applications of stem cell-derived exosomes
[23] https://www.age-regression.com/rir
[24] Personal Communication
Database of Exosomal Proteins; RNA; and Lipids
◉ ExoCarta ~ Exosome, Protein, RNA and Lipid Databas
◉ Vesiclepedia ~ A community compendium for extracellular vesicles and particles





